Deviations from the satisfactory limits prompt more investigation to discover the resource and choose corrective actions.
Lowering the h2o written content has historically been a easy approach to safeguard foods from microbial spoilage. Illustrations in which the available moisture is diminished are dried fruits, syrups, and pickled meats and veggies. Low h2o action may even prevent microbial growth inside of pharmaceutical preparations, see also Sect.
Many virologists consider them as non-dwelling as they absence many of the features of existence, for instance unbiased metabolism. Viruses exist in a variety of states during their lifestyle cycle. Within the extracellular state a virus particle known as a virion.
A single possible strategy to improve the safety of therapeutic biological products is the usage of a virus-retentive filter [23]. Plasma pools may very well be submitted to serological tests and/or genome amplification assays just before They are really released for additional fractionation [24].
The obtained microbial counts are then when compared with predefined acceptance criteria. These requirements are generally recognized dependant on regulatory specifications and enterprise-certain high-quality specifications. If your microbial counts exceed acceptable limits, even more investigation is initiated.
The RE is the ratio of the colony depend on the focus on microorganism recovered to your positive control. It's the popular technique. Typically, a spore-forming microorganism like Bacillus is applied to the material and allowed to dry for this method.
The QC Division must keep abreast of regulatory suggestions check here related to microbial limits in Uncooked resources and finished products.
The information collected including the range website visitors, the resource in which they've come from, and also the internet pages visited in an anonymous type.
Microbial limit test is executed to determine no matter if drug products adjust to an established specification for microbial top quality. Author Identify: Helen
These issues also consider the processing to which the item factors are subjected, The existing technological know-how for testing, and the availability of preferred excellent content. Any of those may preclude the items from distinct necessities beneath Microbial Limit Tests
In the same way, edetate has weak antimicrobial action, and it confers synergistic antimicrobial Attributes when combined with quaternary ammonium substances. On top of that, some active substances may well display considerable antimicrobial activity.
A h2o activity underneath 0.six will not help micro-organisms to expand. Solid oral dosage kinds such as tablets have normally an aw benefit reduce than 0.5 which implies that these products remain steady from a microbiological perspective around here lengthy periods of time Should the item is stored in the water resistant blister that remains integral.
For most products prepared in clinic pharmacies or in institutions like blood banking institutions, the batch measurement is too little (a single or just a few units) or maybe the shelf lifestyle is too short (
Concurrently, the poisonous metabolites of microorganisms and some pathogenic microorganisms also can lead to adverse reactions or secondary infections to clients. As a result, microbial limit testing for non-sterile drug products is probably the vital measures to guarantee the standard, security and efficiency of medication.
 Mr. T Then & Now!
Mr. T Then & Now! Michael Bower Then & Now!
Michael Bower Then & Now! Brandy Then & Now!
Brandy Then & Now! Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now!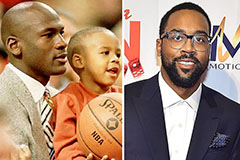 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!